This Phase III study is a global multicenter randomized double-blindplacebo controlled clinical trial to evaluate the efficacy safety and immunogenicity of therecombinant COVID-19 vaccine Sf9 cells in 40000 participants aged 18 years and older who do not have a known history of SARS-CoV-2 infection but whose locations or circumstances put them at appreciable risk of acquiring COVID-19 or SARS-CoV-2. The results of the phase 3 clinical trials of the PfizerBioNTech COVID-19 vaccine candidate have been published in NEJM.
Covid 19 Vaccine These Countries Have Started Rolling Out Coronavirus Vaccine Check Full List Here
All four vaccines given emergency authorization in the US.

Phase 3 trial covid vaccine. It will evaluate the safety and efficacy of NVX-CoV2373 a vaccine. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate Meeting All Primary Efficacy Endpoints Wednesday November 18 2020 - 0659am Primary efficacy analysis demonstrates BNT162b2 to be 95 effective against COVID-19 beginning 28 days after the first dose. PfizerBioNTechsphase three trial began in late July 2020 and the.
INO said that it has expanded partnership with Advaccine Biopharmaceuticals Suzhou Co Ltd. These findings supported progression of the BNT162b2 vaccine candidate into phase 3. The first full peer-reviewed results of phase 3 trials of the COVID-19 vaccine developed by AstraZeneca and Oxford University show that it is safe and up to 90 effective in preventing infection supporting regulatory submissions for emergency use.
Phase 3 clinical trial of investigational vaccine for COVID-19 begins Multi-site trial to test candidate developed by Moderna and NIH. Data are first Phase 3 trial results of a coronavirus vaccine to be published in peer-review literature Today University of Oxford and AstraZeneca researchers present a pooled analysis of Phase 3 trials of a vaccine against SARS-CoV-2 across two different dose regimens resulting in an average efficacy of 704. The Phase 3 study follows the interim Phase 2 results which showed that the adjuvanted recombinant COVID-19 vaccine candidate achieved high rates of neutralizing antibody responses in all adult age groups with 95 to100 seroconversion rates.
Novavaxs two-dose COVID-19 vaccine showed 90 overall vaccine efficacy VE 100 protection against moderate and severe illness and 93 VE against variants of concern and of interest in a phase 3 US clinical trial in adults according to a company news release today. And UKhave publishedresults from the finalphasethree trials. Here we report safety and efficacy findings from the phase 23 part of a global phase 123 trial.
We have already seen the headline results for this vaccine over the past few weeks. Sanofi and GSK initiateglobal Phase 3clinical efficacy study of COVID-19 vaccinecandidate Two-stage design will evaluate vaccine formulations targeting original D614 virus as well as B1351. The Phase 3 trial of another investigational coronavirus disease 2019 COVID-19 vaccine has begun enrolling adult volunteers.
Dr Julian Tang Honorary Associate ProfessorClinical Virologist University of Leicester said. Indias drug regulator has denied Dr Reddys Laboratories permission to conduct Phase 3 trials of the single dose Sputnik Light COVID-19 vaccine in India according to reports. After a single injection high neutralizing antibody levels were also generated in participants with evidence of prior SARS-CoV-2 infection suggesting.
New Delhi India July 1 ANI. RTTNews - Inovio Pharmaceuticals Inc. The subject expert committee SEC did not find any scientific rationale to conduct Phase 3 trials and hence did not consider the application to carry out trials in India The Economic Times reported quoting sources.
Novavax have announced the publication of results from the final analysis of its Phase 3 clinical trial of its COVID-19 vaccine NVX-CoV2373 conducted in the. The randomized placebo-controlled trial will enroll approximately 30000 people at approximately 115 sites in the United States and Mexico. The FDA guidance for Emergency Use Authorization suggests a median duration of follow-up of phase 3 vaccine trial volunteers of 2 months 43.
The central governments Subject Expert Committee SEC has denied permission to Dr Reddys Laboratories for conducting Phase 3 trials of Russias COVID-19 vaccine. Reddys Laboratories has been denied permission by Indias top regulatory body to conduct the Phase-3 trials for the Russian-made Sputnik Light coronavirus disease Covid-19 vaccine. 170 confirmed cases of COVID-19 were evaluated with 162 observed in the placebo group versus 8 in the vaccine.

China S Sinopharm Launches Phase Iii Trial Of Covid 19 Vaccine In Uae
Covid 19 Vaccine China Uae Report Positive Results In Phase 3 Trials All You Need To Know World News Hindustan Times
India S Biological E To Begin Phase Iii Trial Of Vaccine Production From August Reuters

The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson

Fifth Covid 19 Vaccine Reaches Phase 3 Development In Us
Sinopharm S Covid 19 Vaccine Scores Approval In China Pmlive
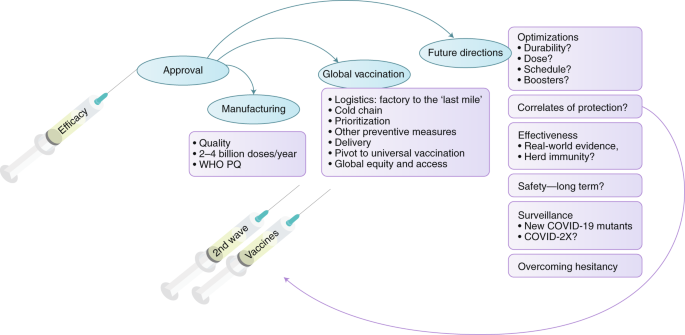
Looking Beyond Covid 19 Vaccine Phase 3 Trials Nature Medicine

J J Seeks Permission To Conduct Phase 3 Trials For Single Shot Covid Vaccine In India Coronavirus Outbreak News

Bahrain Starts Phase Iii Trial Of Sinopharm S Covid 19 Vaccine

Which Covid 19 Vaccine Is Best Here S Why It S Hard To Answer Deccan Herald

Preliminary Phase 3 Trial Results Of The Russian Covid 19 Vaccine Sputnik V News Chemistryviews
Russian Covid 19 Vaccine Sputnik V Under Phase 3 Trials In India To Cost Less Than 10 Per Dose News

Cansino To Conduct Phase Iii Covid 19 Vaccine Trial In Saudi Arabia
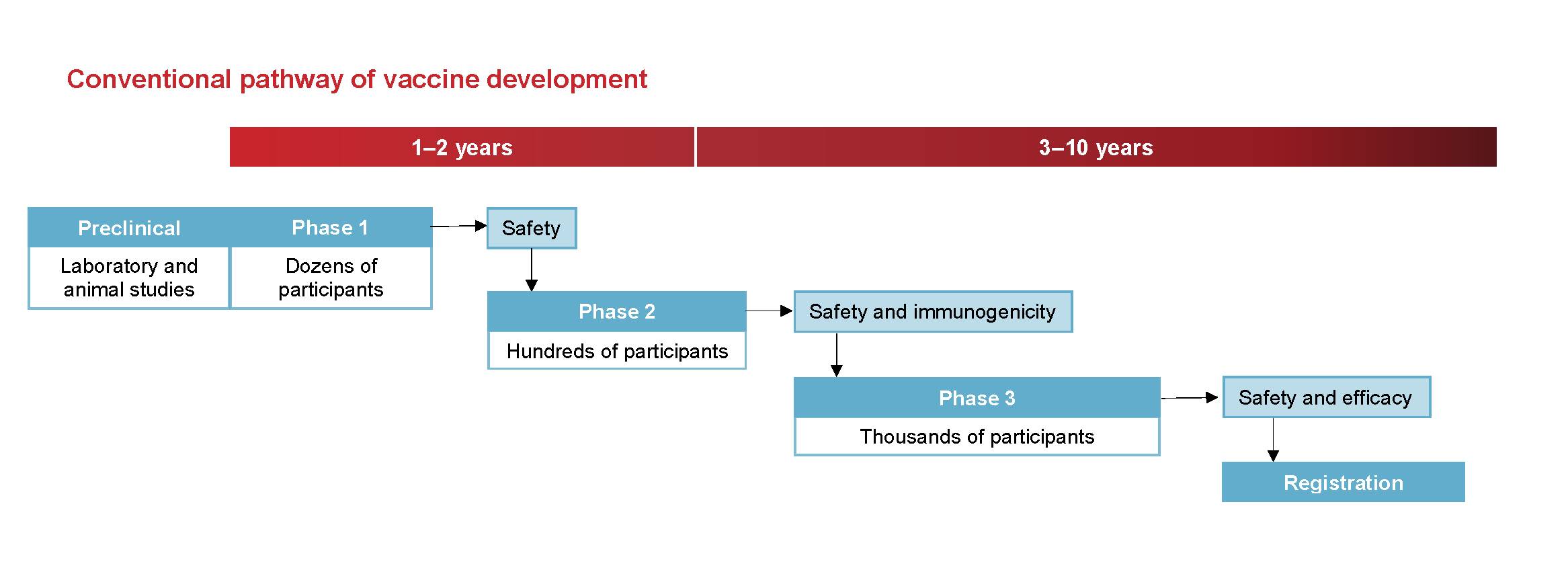
Phases Of Clinical Trials Ncirs

Phase Iii Trial Of Pfizer Biontech Covid 19 Vaccine Starts In South Africa
Pakistan Coronavirus Vaccine Pakistan To Conduct Phase Iii Clinical Trial Of Covid 19 Vaccine Health News Et Healthworld
India S First Covid 19 Vaccine Covaxin Phase 3 Trial Begins At Aiims Key Updates

China Begins Phase I Clinical Trial Of Covid 19 Vaccine
J J Seeks Permission For Phase 3 Trial Of Its Single Shot Covid Vaccine In India Import Licence Times Of India